
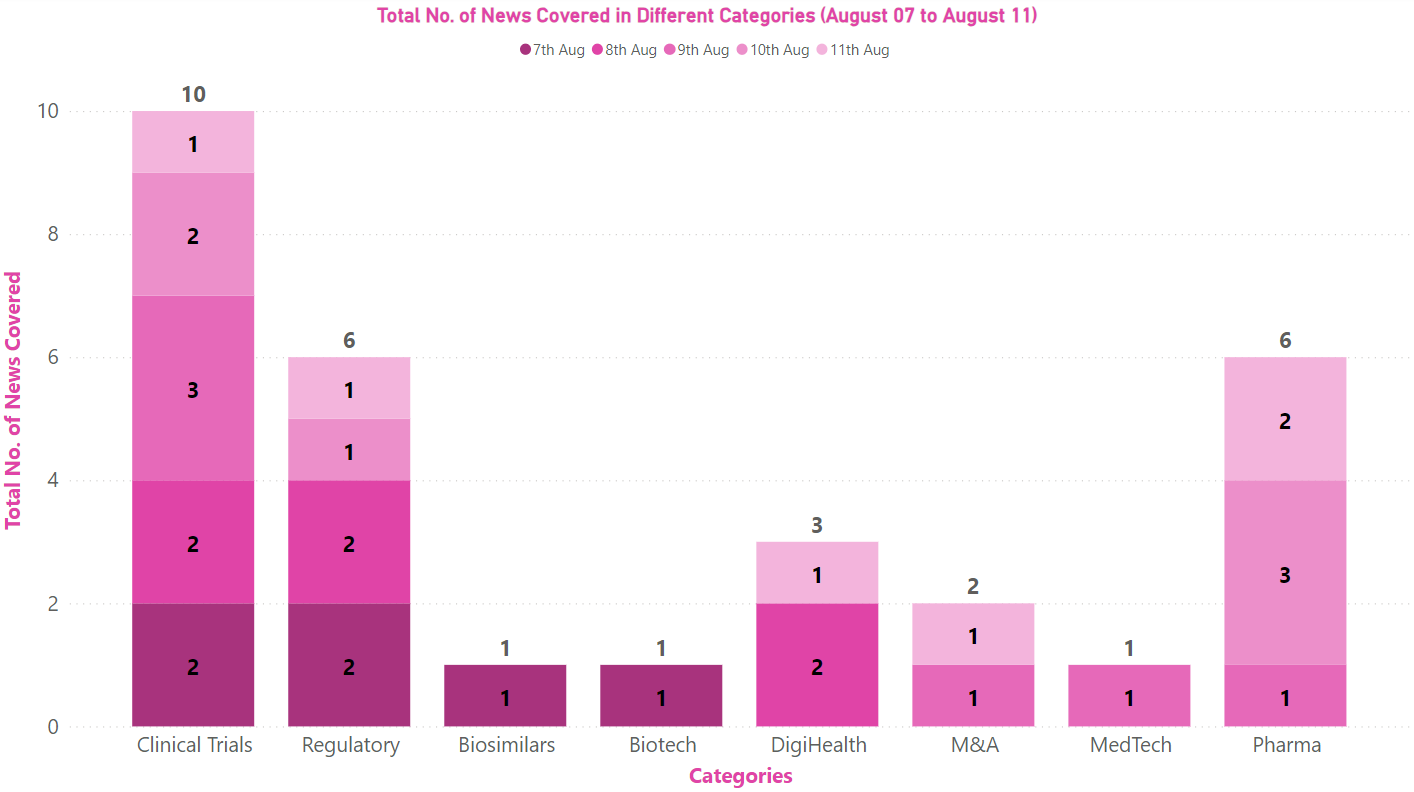
PharmaShots Weekly Snapshots (August 07–11, 2023)
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, M&A, MedTech, and DigiHealth Check out our full report below:

- The US FDA has approved Iveric Bio’s Izervay (avacincaptad pegol intravitreal solution) for the treatment of geographic atrophy, based on the P-III trials (GATHER1 & 2) with a significant reduction in the rate of GA progression
Read more: Iveric Bio
- The US FDA has accepted the NDA of Viatris and Mapi Pharma’s GA Depot for relapsing forms of multiple sclerosis, based on the P-III trial results showed a significant reduction in the annualized relapse rate
Read more: Viatris and Mapi Pharma
- The NMPA has granted BTD for Jacobio Pharma’s Glecirasib to treat pancreatic cancer, based on the clinical efficacy and safety data from ongoing clinical trials
Read more: Jacobio Pharma
- The US FDA has accepted Astellas' sNDA for Cresemba (isavuconazonium sulfate) to treat fungal infections incl. invasive aspergillosis (IA) or invasive mucormycosis (IM) in pediatric patients, based on the P-II study results
Read more: Astellas
- The US FDA has granted accelerated approval to Janssen’s Talvey (talquetamab) for heavily pretreated multiple myeloma & the P-II study (MonumenTAL-1) results showed an ORR of ≥70% with durable responses
Read more: Janssen
- The US FDA has approved Biogen and Sage Therapeutics’ Zurzuvae (zuranolone) for women with postpartum depression, based on the results from the (NEST) clinical development program incl. two studies (ROBIN) and (SKYLARK)
Read more: Biogen and Sage Therapeutics

- Bavarian Nordic highlighted P-III clinical trial results of CHIKV VLP (PXVX0317) for chikungunya virus vaccine in adults and adolescents which was shown to be highly immunogenic in the majority of patients up to 22 days post a single vaccination
Read more: Bavarian Nordic
- Vistagen highlighted P-III (PALISADE-2) trial results of Fasedienol (PH94B) nasal spray for social anxiety disorder meeting primary & secondary efficacy EPs
Read more: Vistagen
- Telix dosed the first patient in the P-I study (IPAX-2) of TLX101 for newly diagnosed glioblastoma in combination with external beam radiation therapy & temozolomide
Read more: Telix
- Terns Pharmaceuticals highlighted P-IIa trial (DUET) results of TERN-501 for NASH meeting 1EPs & 2EPs with dose-dependent MRI-PDFF reductions
Read more: Terns Pharmaceuticals
- UroGen Pharma highlighted P-III trial (ATLAS) trial results of UGN-102 for low-grade, intermediate-risk non-muscle invasive bladder cancer showing superiority to TURBT with a 55% reduction of risk for recurrence
Read more: UroGen Pharma
- Novartis’ remibrutinib met their primary endpoints in the P-III trials and showed rapid symptom control in chronic spontaneous urticaria along with rapid clinical improvements across urticaria disease activity scores
Read more: Novartis
- Regeneron highlighted 2yr. (PULSAR) trial results of Aflibercept for wet age-related macular degeneration demonstrated durable vision gains at extended dosing intervals
Read more: Regeneron
- Palisade Bio highlighted the P-II (PROFILE) study results of LB1148 for post-surgical abdominal adhesions failed to meet its primary efficacy EPs of reducing adhesions
Read more: Palisade Bio
- Pliant Therapeutics initiated a P-IIb (BEACON-IPF) trial of Bexotegrast for idiopathic pulmonary fibrosis, following positive data from the P-IIa trial (INTEGRIS-IPF)
Read more: Pliant Therapeutics
- Eli Lilly highlighted the P-III study (LIBRETTO-431) results of Retevmo (selpercatinib) for newly-diagnosed advanced or metastatic RET fusion+ NSCLC, which showed a significant clinical improvement in PFS
Read more: Eli Lilly

- Formycon and Fresenius Kabi entered into a settlement agreement with Johnson & Johnson for FYB202, a proposed ustekinumab biosimilar in the US
Read more: Formycon and Fresenius Kabi

- DualityBio & BioNTech expand their partnership to develop, manufacture and commercialize third antibody-drug conjugate therapeutics for solid tumors. The recent agreement was based on the previous Apr 2023 collaboration
Read more: DualityBio & BioNTech

- The US FDA has cleared Abbott’s Alinity H-Series Hematology system enabling laboratories to run complete blood counts
Read more: Abbott
- Nexalin Technology’s Gen-2, 15 Milliamp neurostimulation device showed a significant benefit & reduction in frequency & degree of pain in migraine patients
Read more: Nexalin Technology
- Acorai earned Breakthrough Device Designation from the US FDA for intracardiac pressure monitor
Read more: Acorai

- Amarin and Neopharm collaborated to commercialize Vazkepa (Icosapent Ethyl) in Israel, following recent regulatory approval in Israel
Read more: Amarin and Neopharm
- Antengene & Hansoh Pharma collaborated for Xpovio (selinexor) in the Mainland of China & seeks to make Xpovio available to the widest possible number of Chinese patients with hematological malignancies
Read more: Antengene & Hansoh Pharma
- Daiichi Sankyo launches Vanflyta (quizartinib) in the US for newly diagnosed FLT3-ITD positive AML & showed 22% reduction in risk of death in the P-III (QuANTUM-First) trial
Read more: Daiichi Sankyo
- Fresenius Kabi launches Plerixafor Injection, a generic equivalent to Mozobil in the US
Read more: Fresenius Kabi
- Corium & Lotus Pharmaceutical collaborated for Adlarity to treat Alzheimer's Disease in ten markets across Asia
Read more: Corium & Lotus Pharmaceutical
- Arcutis collaborated with Huadong to develop, manufacture, and commercialize topical roflumilast in Greater China (mainland China, Hong Kong, Macau, and Taiwan) and Southeast Asia for multiple dermatological conditions
Read more: Arcutis & Huadong

- Regeneron to acquire Decibel Therapeutics to strengthen gene therapy and hearing loss programs
Read more: Regeneron & Decibel Therapeutics
- Addimmune to go Public via SPAC deal with 10x Capital Venture Acquisition Corp. III for ~$500M while the combined company is expected to trade under the ticker symbol “HIV”
Read more: Addimmune

- The US FDA has approved Boston Scientific’s POLARx cryoablation system for paroxysmal atrial fibrillation, based on (FROZEN-AF IDE) trial results that demonstrated the safety and effectiveness
Read more: Boston Scientific
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














